Transition of RSV Vaccine Ordering to NCIR
Effective Tuesday, October 21, 2025, the NCIP is transitioning RSV vaccine ordering from the Smartsheet Request Form to the North Carolina Immunization Registry (NCIR).
Note: All orders previously placed through Smartsheet have been processed.
Inventory Management
Please monitor your inventory and maintain sufficient stock to meet the needs of Vaccines for Children (VFC)-eligible patients throughout the 2025–2026 respiratory season.
Ordering Considerations
- If allocations are out, RSV vaccine orders may be held or declined until more doses become available. We do not anticipate shortages this season. We receive an allocation of doses from CDC bi-weekly. Order smaller orders more frequently to avoid surpassing the routine allocation.
- If you request ENFLONSIA™ (Clesrovimab) in 10-packs and supply is unavailable, your order may be fulfilled with 1-pack units.
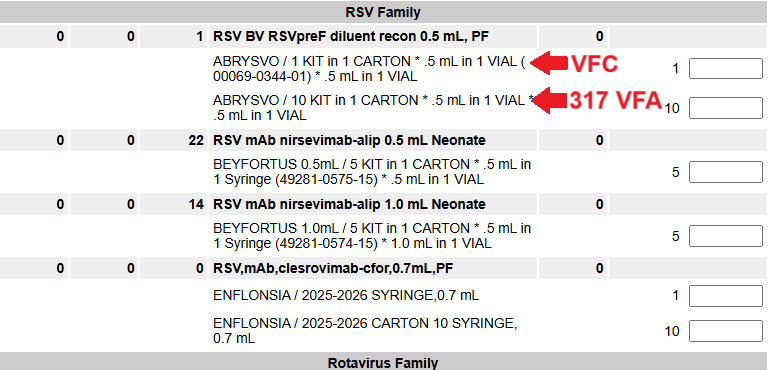
- When ordering maternal ABRYSVO®, VFC ABRYSVO® is only available in the 1 KIT in 1 CARTON .5mL in 1 VIAL (NDC 00069-0344-01) and 317 VFA is only available in the 10 KIT in 1 CARTON .5mL in 1 VIAL (example below). As a reminder, these doses are only available for 317-eligible pregnant adults or VFC-eligible pregnant people under age 19.
COVID-19 Ordering Updates
Below is the anticipated shelf life of COVID-19 vaccines to help with order planning. To reduce waste, CDC recommends smaller, more frequent orders—ideally quantities that can be used within 2 weeks, and no more than a 4–6 week supply.
- Moderna: Providers may receive vaccine with less than 7 months of shelf life. Initially, expect dating between 3 to 6 months; as the season progresses, shelf life may be shorter. Distribution will generally stop when vaccines have 28–30 days of shelf life remaining, unless otherwise communicated. Shelf life may shorten later in the season.
- Pfizer:
- Ages 12+: Shipped refrigerated with at least 12 weeks' shelf life.
- Ages 5–11: Shipped ultracold with at least 3 months shelf life (when kept at ultracold temps).
- Sanofi/Novavax: Vaccine is received at CDC’s depots with at least 2 months of shelf life. Early season shipments expire Dec 31, 2025. Shelf-life extensions are pending FDA approval and will be communicated. Distribution typically ends with 14 days remaining, unless otherwise communicated.
We will continue to monitor the immunization landscape and communicate any future updates as they become available. We appreciate your continued partnership and patience during this transition.
How to contact us:
For assistance, please contact the NCIP Help Desk by phone at 1.877.USE.NCIR (1-877-873-6247) or by email.
Thank you for your ongoing partnership and dedication to improving immunization outcomes in North Carolina.
In Health,
NC Department of Health and Human Services
